Visit our Research site
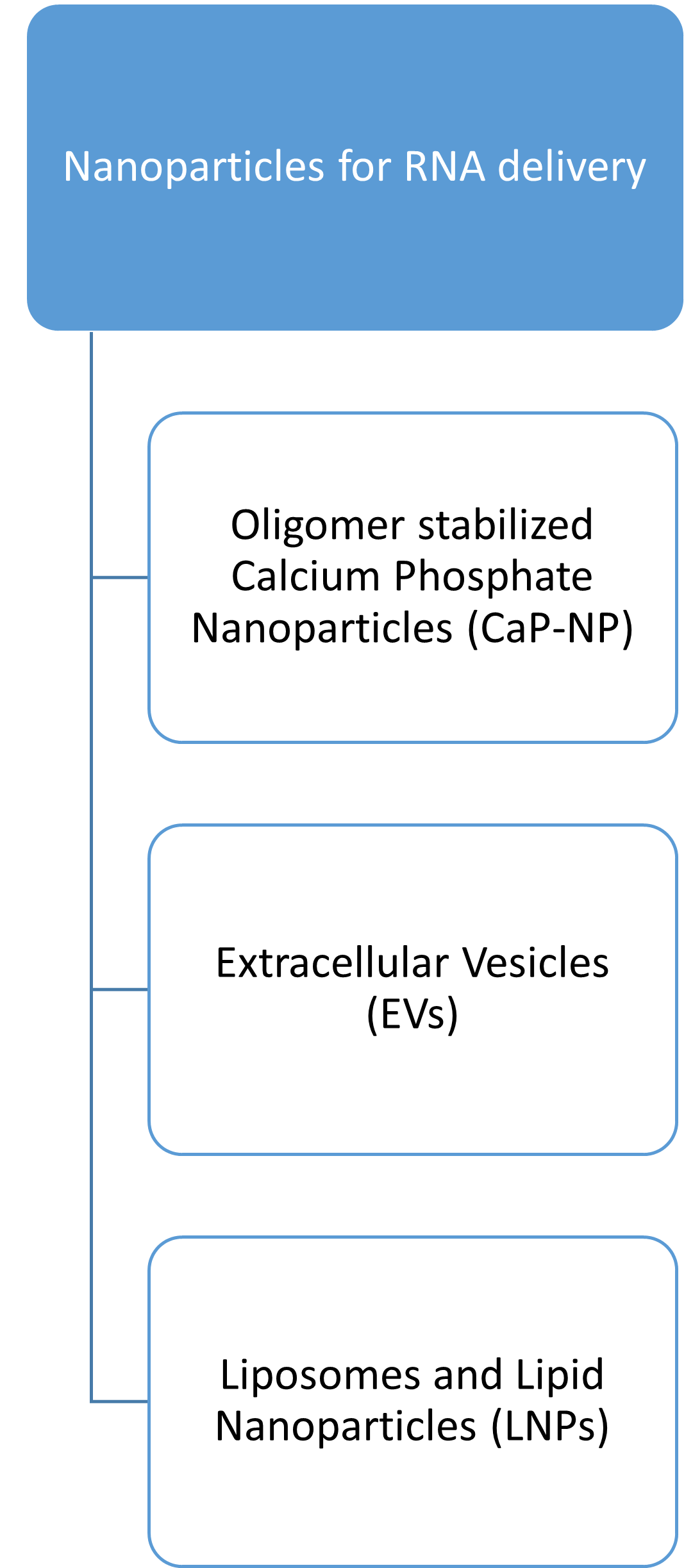
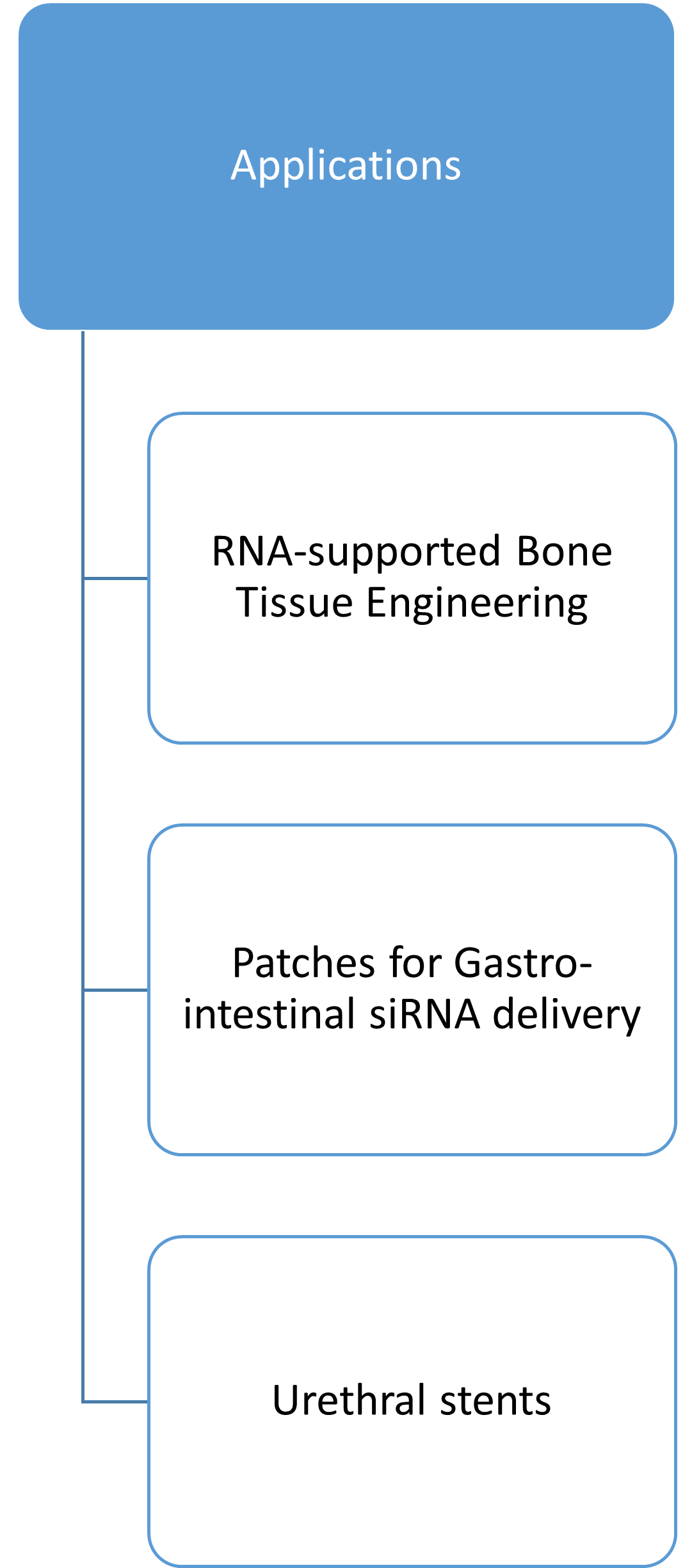
The pharmaceutical technology in Leipzig has a lot to offer. Visit our subsites by clicking on the pictures below to find out about our research and scientists!

funding
We kindly thank our funding partners for supporting our research.