Glial diversity in chronic peripheral nerve disorders
Demyelinating neuropathies are characterized by primary defects of Schwann cells which lead to slowly progressive demyelination and secondary axonal loss that underlies clinical impairment. Unfortunately, no causal therapy is available for the vast majority of demyelinating neuropathies, as the pathological mechanisms remain poorly understood. In previous studies we observed that individual Schwann cells display very different morphological and molecular characteristics in demyelinating polyneuropathies. In this diseases, myelin disassembly is indeed not a concerted process of all Schwann cells, but occurs rather focally in individual cells. This fascinating observation has been described more than 60 years ago and is referred to as segmental demyelination that explains the slowly progressive nature of the disease (Figure 1).
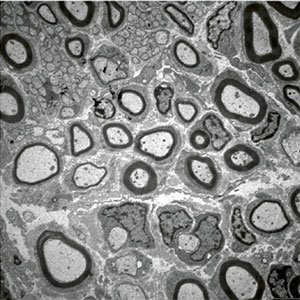
Figure 1: Electron microscopic picture of a cross section through a neuropathic nerve showing axonal fibers engulfed by Schwann cells myelin (dark rings).Some fibers are properly myelinated (thick rings) whereas others are de-myelinated.
Why do individual Schwann cells disintegrate whereas others aligned with the very same nerve fiber remain intact? To answer this question we chose to combine new molecular and imaging techniques to isolate and characterize specific Schwann cell populations that are prone to degeneration in mouse models for demyelinating polyneuropathies. The goal is to unravel the initial mechanisms of disease manifestation that are responsible for the subsequent process of demyelination. Vice versa, we aim at a better understanding of the repair mechanisms in peripheral nerves, given the fact that Schwann cells are in principle able to remyelinate demyelinated segments. However, Schwann cells lose their ability to reconstitute the original myelin sheath length during remyelination and consequently nerve function is only partially restored. By applying surgical and genetic techniques in mice, we investigate the mechanisms of longitudinal myelin growth and the reassembly of myelin sheath segments in chronic nerve disorders. Here, both, longitudinal and radial myelin growth require an enormous metabolic effort, especially with regard to lipids, which constitute about 70% of the myelin sheath (Figure 2).
Figure 2: Cell culture of neurons and Schwann cells. Neuronal fibers (red) are enwrapped by Schwann cells (nuclei in blue) that produce myelin (pink). Phospholipids (green) are a constituent of the myelin sheath.
Next to the Schwann cell intrinsic biosynthesis of lipids, circulating lipids appear to play an important role for Schwann cell function and systemic dyslipidemia has been suggested to play a central role in the pathomechanism of diabetic polyneuropathy. How do Schwann cells integrate lipid-associated Signals? To address this question we characterize mouse models for peripheral neuropathies with altered systemic lipid homeostasis. In addition, we genetically manipulate regulatory pathways in Schwann cells that are linked to lipid metabolism. With the intended approaches it is not only our goal to gain insight into the profound disease and repair mechanisms of demyelinating neuropathies, but also to contribute to the development of new therapeutic strategies for this life-burdening diseases (Figure 3).
Figure 3: Nerve cross sections of wiltype rats (left panel) and neuropathic rats that display demyelination (less blue myelin rings, middle panel). Neuropathic rats that were treated with a phospholipid-enriched diet display improved myelination (right panel).