Project 1: Role of macrophages in adipocyte degadation - a live-imaging approach
Martin Gericke (Institute of Anatomy Leipzig)
Funded by DFG Collaborative Research Centre 1052 "Obesity Mechanisms"
Innate immune cells, such as macrophages, degrade dying cells or cell debris, a process called efferocytosis. Efficient efferocytosis licenses anti-inflammatory removal of aged or damaged cells and is crucial to maintain tissues homeostasis. In adipose tissue (AT), clearing of dead adipocytes seems to be challenging, because hypertrophic adipocytes in obesity can exceed more than 150 µm in diameter, a dimension about 10-fold bigger than regular phagocytes. To investigate the molecular and cellular events following adipocyte death, we have recently established a live-imaging approach allowing us to study adipocyte - macrophage interaction after adipocyte death in living tissue. Hence, we are aiming at determining mechanisms for efficient and inefficient efferocytosis of adipocytes using our live-imaging approach combined with subsequent post-hoc super-resolution microscopy or electron microscopy. The combination of these techniques can further determine the molecular interaction between macrophages and dying adipocytes in an unprecedented spatio-temporal resolution.
This project is designed to i) unravel the molecular decision process in macrophages leading to recognition and engulfment of adipocyte remnants and, ii) study if a critical size is a natural limit for efficient, non-inflammatory removal of large particles, such as hypertrophic adipocytes in obesity. Our working hypothesis is that inefficient efferocytosis of hypertrophic adipocytes leads to a pro-inflammatory microenvironment and type 2 diabetes. Therefore, increasing scavenger function of macrophages could be a new pharmacological target to treat metabolic disease.
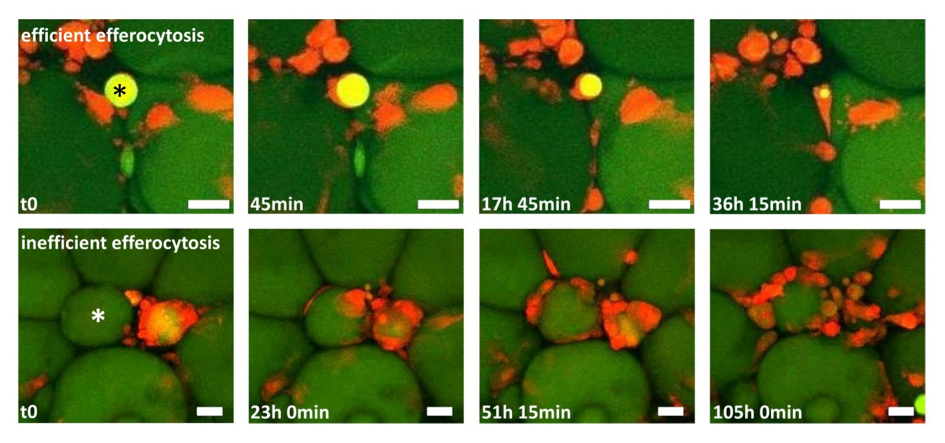
Figure 1: Two time-lapse movies unravel fundamentally different clearing behaviour of macrophages in adipose tissue (AT). AT explants showing macrophages (red) and adipocytes (green). Upper row: One macrophage detects, internalizes and digests a small adipocyte remnant (star), a process leading to efficient removal of the adipocyte remnant within ~36 h. Lower row: After initial contact between macrophages and a hypertrophic adipocyte (star), more macrophages become attracted, resulting in a CLS formation with now signs of efficient degradation after 4 days. Scale bar represents 50 µm.
Project 2: Effect of intermittent fasting on obesity-induced carcinogenesis / Auswirkungen des intermittierenden Fastens auf die Adipositas-assoziierte Pankreaskarzinogenese
Martin Gericke (Institut für Anatomie, Leipzig)
Funded through DFG-RTG 2751 "Inflammatory cues as modulators of early pancreatic carcinogenesis” (InCuPanC)
Obesity is frequently associated with chronic inflammation in several organs, including the pancreas (pancreatitis). Most importantly, obesity and chronic pancreatitis are well-established risk factors for pancreatic cancer. Interestingly, intermittent fasting decreases the incidence of obesity-associated cancer. Whether intermittent fasting also decreases obesity-associated carcinogenesis in the pancreas is still elusive. Further, the molecular mechanism remains to be defined. We here investigate, whether alternatively-activated (so-called M2) macrophages mediate the diet-dependent effects on pancreatic inflammation and early carcinogenesis. Of note, pancreatic inflammation and fibrosis depend on interleukin-4 (IL-4) signaling on macrophages, which is mandatory for alternative activation or M2 polarization of resident macrophages. Further, intermittent fasting increases the number of M2 macrophages in the adipose tissue, which has been proposed to mediate beneficial effects. Therefore, this project is designed to unravel the effects of intermittent fasting on obesity-associated pancreatic carcinogenesis and evaluate the role of M2 macrophages in this process.