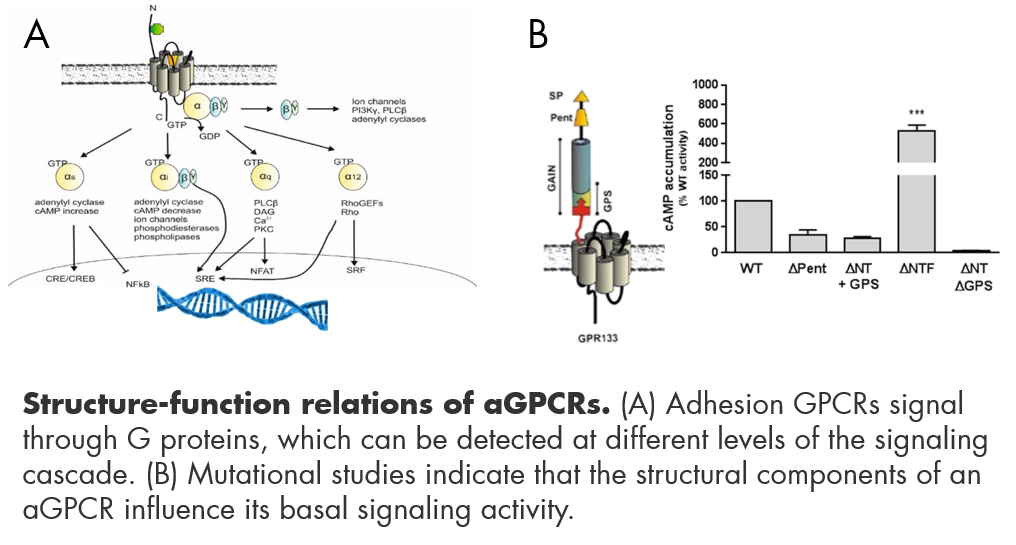
1) Structure/function relations in aGPCR signal transduction
Adhesion GPCRs are highly complex molecules composed of a diverse set of functional domains, which are encoded by several exons. As such they are subjected to splicing, which yields multiple different receptor proteins. These receptor variants can lack N-terminal domains or even transmembrane domains (Knierim et al. Sci Rep. 2019). The functional testing of a large set of receptor mutants reveals significant influence of the N-terminal domains on basal signaling levels. The splicing events appear to have tissue preferences, thus allowing for a broader range of functional regulation (Wilde et al. Faseb 2016). Further, numerous single nucleotide polymorphisms have functional implications for aGPCRs (Fischer et al. BMC Genomics 2016). To elucidate the role of structural components on signal transduction cascades, ligand binding and activation levels of aGPCRs we are using an overexpression approach of mutant vs wildtype receptors in heterologous cell systems. Intracellular signaling pathways are studied using second messenger accumulation assays, ELISA techniques, luciferase reporters, Western Blot or FRET/BRET. We further aim to identify the points of interaction between the tethered agonist and its binding pocket using biochemical and computational approaches.
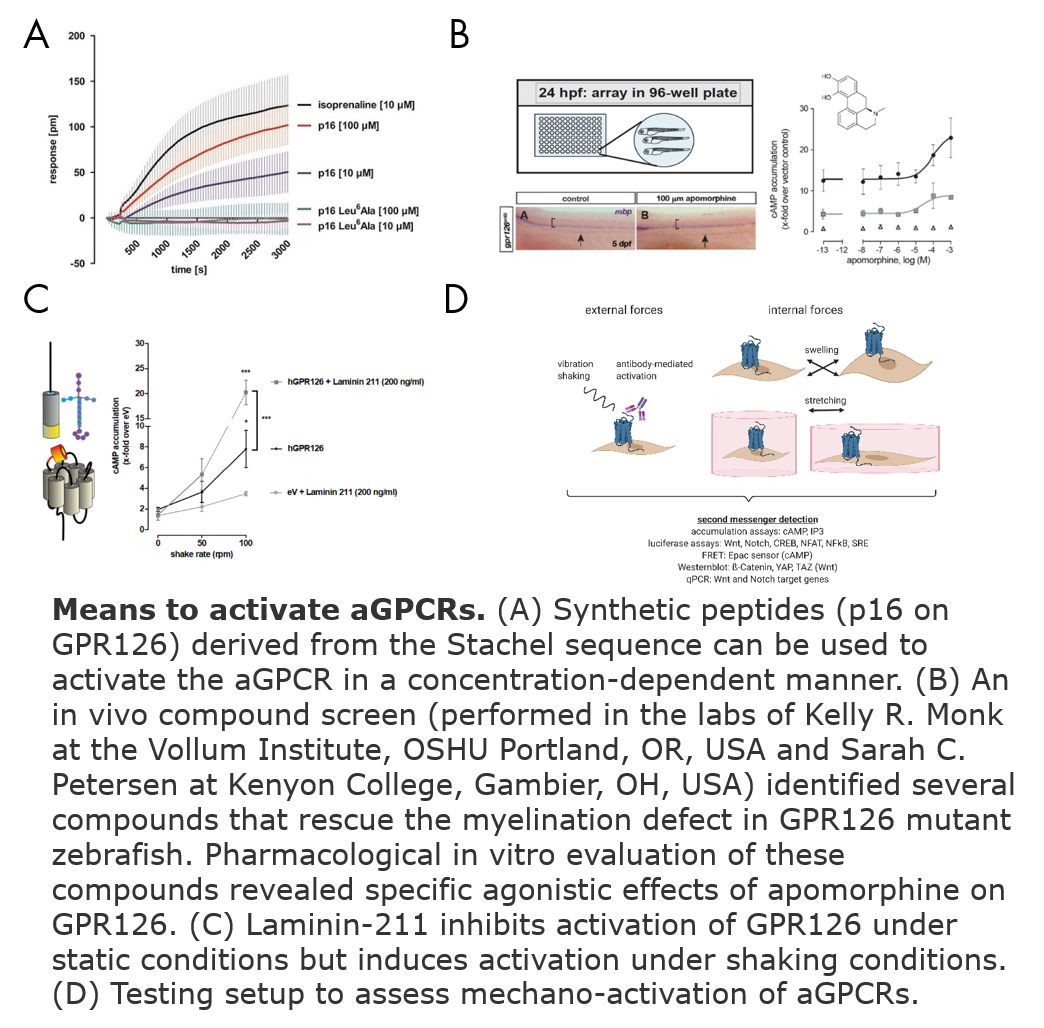
2) Means of aGPCR activation (Ligand identification, mechanical stimulus, small molecule compounds)
Modifying aGPCR activation levels is the basis to study their function in vitro and in vivo. Upon identification of a tethered agonist sequence within the receptor, we established derived peptides as agonists and antagonists (Liebscher et al. Cell Rep. 2014, Demberg et al.BBRC 2015, Wilde et al. Faseb 2016, Demberg et al. JBC 2017). These peptides, however, have several disadvantages e.g. low affinity, bad solubility and unspecific activation of several aGPCRs. Thus, there is a need for alternate ways to modulate aGPCR activity. Thus, we are pharmacologically characterizing small molecule compounds that have been identified in in vivo (Bradley et al. ANYAS 2019) as well as in silico drug screens. Further, we test potential ligands, which are mainly components of the extracellular matrix, for their impact on receptor signal transduction (Petersen et al. Neuron 2015). We could show that aGPCRs can be activated by different mechanical stimuli, e.g. vibration, shaking or cell swelling. We are currently seeking to characterize these forces that act on the receptor.
3) G protein-dependent and independent intracellular pathways
The majority of aGPCRs have been shown to functionally couple to G proteins. However, interaction of the C-terminus with the cytoskeleton has been shown (Hilbig et al. Cell Rep. 2018). There is increasing evidence that aGPCRs can also signal through other G protein-independent pathways like Wnt or sonic hedgehog (shh). We aim to characterize these interactions and to identify other intracellular binding partners that could convey so far unknown functions of aGPCRs.
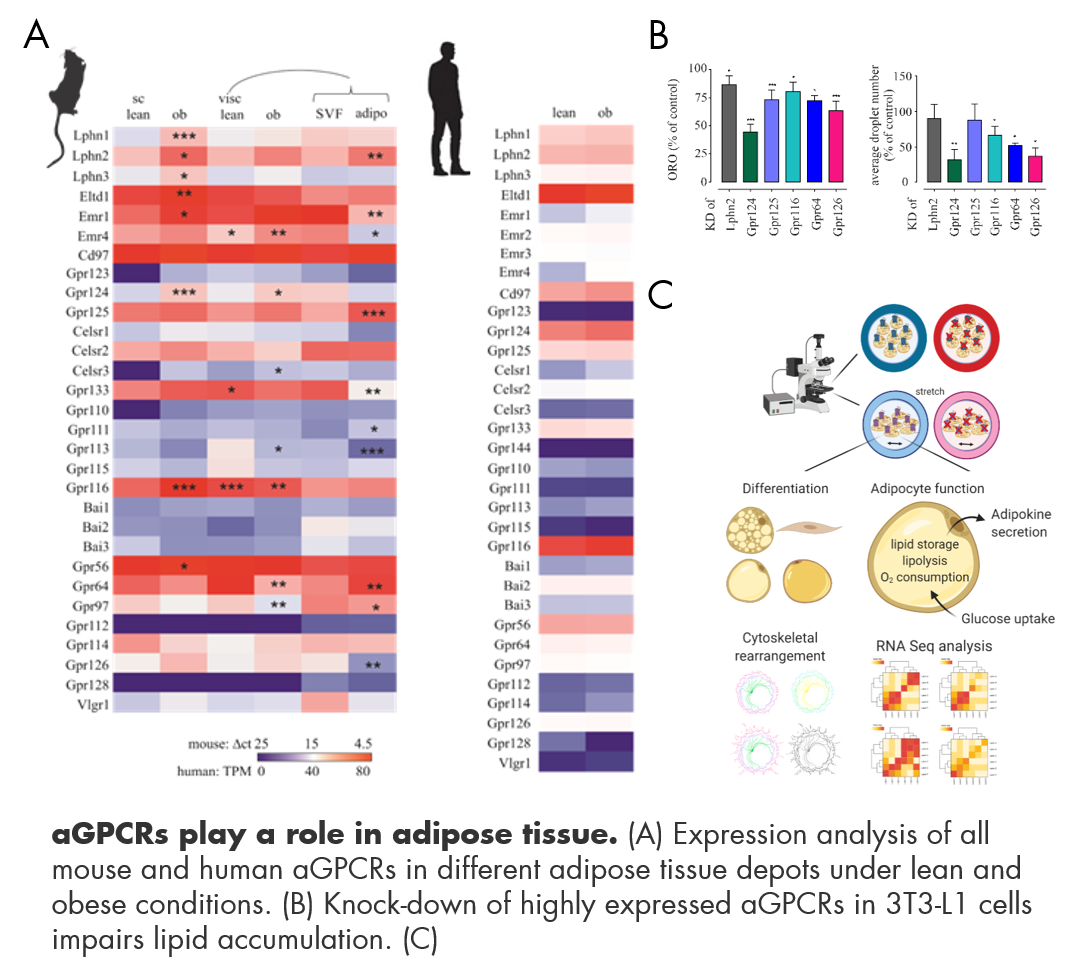
4) The role of aGPCRs in tissue function
As aGPCRs act as mediators or sensors of their immediate surrounding, it is of great interest to us to elucidate their role in a specific tissue environment, such as the function of CD97 in retinal pigment epithelium cells (Eichler et al.ANYAS 2019), or the role of aGPCRs in adipose tissue. We established the repertoire of a mouse and human aGPCRs in different adipose tissue depots under normal and high fat diet conditions (Suchy et al. Int J Obes 2020). We identified several aGPCRs that are decisively contributing to differentiation of 3T3-L1 cells, which is a model cell line for adipocyte function. Our current focus is to investigate the influence of mechanical force on aGPCR function in adipocytes. To mimic the physiological forces that can occur in adipose tissue through hypertrophy and hyperplasia of expanding adipocytes as well as the potential forces mediated by changing extracellular matrix compositions, we use cell swelling or cell stretch applied by a Fexcell Tension® system. We are further interested in the role of aGPCRs in fibroblasts, muscle and bone cells.
5) The physiological role of aGPCRs in mouse and zebrafish with implications for human diseases
GPCRs are among the most important drug targets. Thus, identifying the physiological role of the orphan class of aGPCRs will help to unravel their contribution to diseases, which forms the basis of establishing them as new therapeutic targets. We investigate the consequences of receptor knock-out (KO) in mice in order to identify potential human phenotypes that might be caused by mutations in the receptor gene. Once physiological impairments have been assessed, genome wide association studies and human cohorts will be screened to identify affected human individuals. To further delineate the molecular causes of each phenotype we employ tissue-specific KO mice and zebrafish as an easy-to-target and observable animal model.
Our current projects involve:
- The role of GPR133 in cardiac dysfunction
- The role of GPR133 in metabolism
- The role of GPR133 in bone homeostasis
- The role of GPR133 in spine development
- The role of GPR114 in immune response